酸化ストレスがDNAのテロメアの短縮(老化)を促進することが立証された
- 2019.06.16
- 学会・論文

原本https://www.upmc.com/media/news/051419-opresko-rosより
目次Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis
Highlights
- •
Targeted chronic 8-oxoG damage at telomeres promotes telomere shortening- •
Unrepaired telomeric 8-oxoG in OGG1-deficient cells impairs telomere replication- •
8-oxoG-induced telomere losses cause dicentric chromosomes and anaphase bridges- •
Persistent telomeric 8-oxoG drives telomere crisis and global genomic instabilitySummary
Telomeres are essential for genome stability. Oxidative stress caused by excess reactive oxygen species (ROS) accelerates telomere shortening. Although telomeres are hypersensitive to ROS-mediated 8-oxoguanine (8-oxoG) formation, the biological effect of this common lesion at telomeres is poorly understood because ROS have pleiotropic effects. Here we developed a chemoptogenetic tool that selectively produces 8-oxoG only at telomeres. Acute telomeric 8-oxoG formation increased telomere fragility in cells lacking OGG1, the enzyme that removes 8-oxoG, but did not compromise cell survival. However, chronic telomeric 8-oxoG induction over time shortens telomeres and impairs cell growth. Accumulation of telomeric 8-oxoG in chronically exposed OGG1-deficient cells triggers replication stress, as evidenced by mitotic DNA synthesis at telomeres, and significantly increases telomere losses. These losses generate chromosome fusions, leading to chromatin bridges and micronucleus formation upon cell division. By confining base damage to the telomeres, we show that telomeric 8-oxoG accumulation directly drives telomere crisis.

https://logmi.jp/business/articles/321383
ピッツバーグ大学が『Molecular Cell』誌上で新たに発表した研究で、
酸化ストレスが老化を促進することが立証された
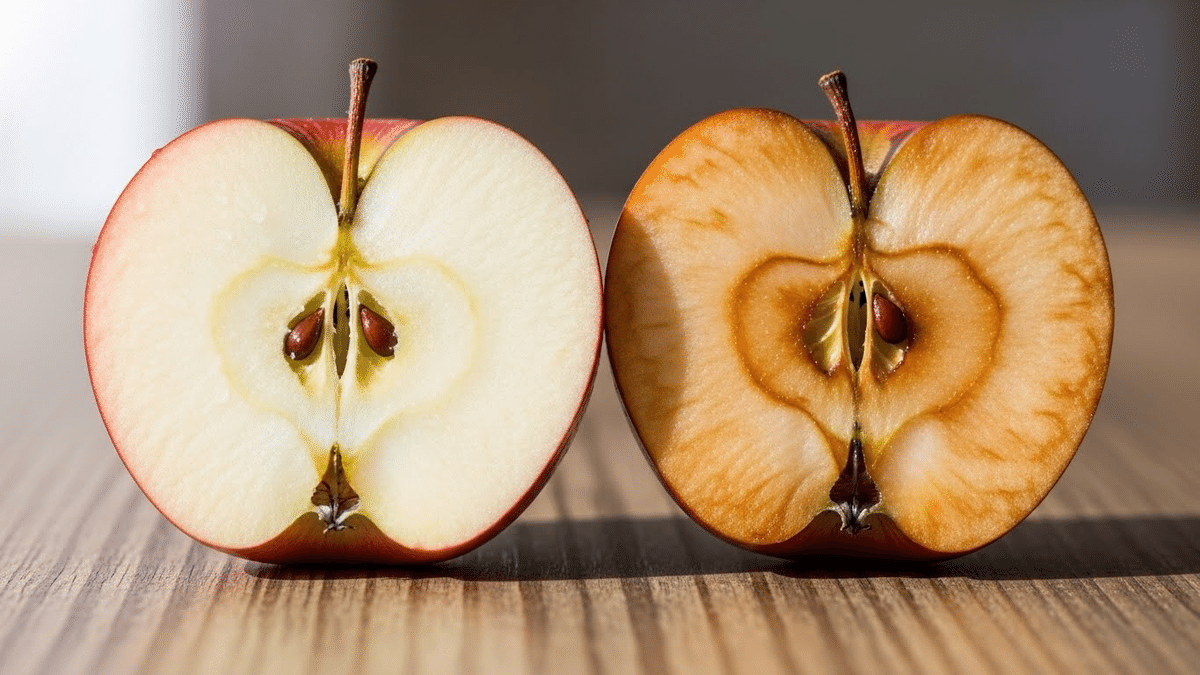
人体の細胞生物学では以前から、染色体の末端を覆うテロメアという部位に、酸化ストレスが影響を及ぼすことがわかっていました。
テロメアが無いDNAの末端は、細胞同士がくっついてしまい、細胞分裂の際に、染色体の正常な分裂が妨げられてしまいます。つまりテロメアが無いと、ゲノムはスパゲッティ状態にからまってしまうのです。
「テロメアは細胞分裂のたびに短くなる」。
どんなに健康な細胞であっても、テロメアは短くなり続け、しまいには細胞分裂ができなくなります。研究者たちは、これこそが体内の器官を老化させる基本構造だと考えています。
24時間、酸化ストレスにさらされた状態を疑似再現したところ、一貫してテロメアの短化が認められました。長時間ストレス下に置かれたこれらのグループでは、テロメアが短化した染色体は癒着を起こし、スパゲティ状態になってしまいました。つまり、染色体の自己複製力に影響を及ぼすことがわかったのです。
https://www.eurekalert.org/pub_releases/2019-05/uop-psf050819.phpより
Pitt study finds direct oxidative stress damage shortens telomeres
PITTSBURGH, May 14, 2019 – The same sources thought to inflict oxidative stress on cells–pollution, diesel exhaust, smoking and obesity–also are associated with shorter telomeres, the protective tips on the ends of the chromosomal shoelace.
A new study from the University of Pittsburgh, published today in Molecular Cell, provides the first smoking gun evidence that oxidative stress acts directly on telomeres to hasten cellular aging.
“Telomeres consist of hundreds of guanine bases, which are sinks for oxidation,” said senior author Patricia Opresko, Ph.D., professor of environmental and occupational health at the Pitt Graduate School of Public Health and UPMC Hillman Cancer Center. “Is it just a coincidence? Or could it be true that oxidizing those guanines in the telomeres is really contributing to shortening?”
To find out for sure, Opresko needed some way to inflict oxidative stress on telomeres and nowhere else.
So, she enlisted the help of Marcel Bruchez, Ph.D., professor of biological sciences and chemistry and director of the Molecular Biosensors and Imaging Center at Carnegie Mellon University. Bruchez developed a method for zeroing in on the telomeres using a special light-activated molecule that latches onto the telomere and delivers localized free radicals–the molecular agent of oxidative stress–on command.
“One of the main challenges to targeting oxidative damage to specific loci in living cells has been achieving precise temporal and dose-control of this damage,” Bruchez said. “By combining telomere targeting with our optochemogenetic generation of singlet oxygen, we are able to selectively control when and how hard the oxidative stress is applied specifically at the telomere sites.”
The researchers repeatedly exposed cultured cancer cells to this targeted oxidation procedure, mimicking conditions of environmental oxidative stress and inflammation, and, indeed, they saw the telomeres break and shorten with each cell division, despite repair efforts by the telomere lengthening enzyme telomerase.
As the DNA repair machinery tried to fix the broken telomeres, the ends of the chromosomes often fused together, destabilizing the genome and preventing cells from dividing properly.
Whereas telomere shortening spells bad news for healthy cells, Opresko said, the flipside is that targeting telomeres might offer a way to fight cancer. With short enough telomeres, cancer cells would stop dividing.
“If we can understand what causes telomere shortening and how cells compensate for that,” Opresko said, “then we’ll be in a better position to design intervention strategies that protect telomeres in healthy cells and target telomeres in cancer cells.”
###
Other authors on this study include Elise Fouquerel, Ph.D., Ryan Barnes, Ph.D., Shikhar Uttam, Ph.D., and Simon Watkins, Ph.D., all of Pitt.
This work was supported by grants from the National Institutes of Health (K99ES027028, R01ES022944, R01CA207342, R01ES02842, R21/R33ES025606 and R01EB017268).
To read this release online or share it, visit http://www.
upmc. com/ media/ news/ 051419-opresko-ros.








 サイクルイオン
サイクルイオン MiaSole FLEXシリーズ
MiaSole FLEXシリーズ




